GRAPHENE - THE PHYSIOCHEMICAL WONDER
By Vedant Nair
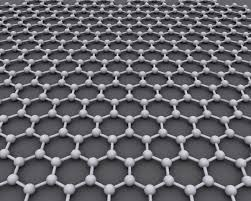
Graphene is an allotrope of carbon, which means that it’s a special arrangement of atoms made entirely of carbon. It’s arranged in a hexagonal lattice, so each carbon atom is attached to three others with double bonds, and each layer is one atom thick.
Graphene was discovered as a theoretical innovation in the late 1940s with the development of graphite, and only came into practical use decades later. Graphite is another allotrope of carbon, which is essentially multiple layers of graphene. Although it was thought of then, and scientists attempted to mechanically produce it in the 1990s, it was first successfully produced in 2004 by two scientists from the University of Manchester.
Graphene took a significantly longer time to produce than graphite because it was incredibly hard to isolate a single layer of carbon atoms. The way it was produced in 2004 was by hours of using tape to separate the carbon atoms on a dot drawn by a graphite pencil. The ingenuity behind it is amazing and although it initially took an immense effort to successfully produce graphene, it is a truly awesome material. Even though it isn’t a metal, due to its delocalised electrons, it can conduct electricity, and above of all, it’s exceptionally strong. Because of these valuable abilities, graphene has multiple applications.
Firstly, using the delocalised electrons, graphene can be used to make better batteries. The current lithium ion (li-ion) batteries have slow recharge times, can explode and are too big and rigid for future technology to be smaller and more portable. Graphene can fix this easily. Being only one atom thick, multiple, separated disks of graphene can be stacked to hold charge. Additionally, being so thin allows layers of graphene to be malleable. This means batteries could be built that could change shape in accordance to flexible tech, including wearable technology.
The prospect of wearable technology is enhanced by graphene’s use in displays. Again, graphene’s thinness plays a crucial role. Gaps between the carbon atoms can allow light to effortlessly pass through and the strength of the carbon-carbon double covalent bonds could make graphene displays immensely stronger than any glass or plastic displays used in today’s technology.
By and large, graphene is the future for technology. Both as a fundamental building material and to use inside, as part of the structure. The fabrication of graphene does currently face some issues and the cost of production is high. Current research is dedicated to efficient and cheap methods to make graphene, but it’s just a matter of time before graphene dawns upon the scientific world as a mainstream material.